Dissecting the steps of CO2 reduction: 2. The interaction of CO and CO2 with Pd/γ-Al2O3: an in situ FTIR study†
Abstract
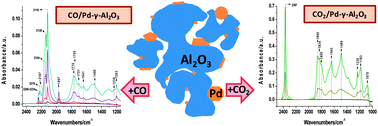
Alumina supported Pd catalysts with metal loadings of 0.5, 2.5 and 10 wt% were investigated by in situ FTIR spectroscopy in order to understand the nature of adsorbed species formed during their exposure to CO2 and CO. Exposing the annealed samples to CO2 at 295 K resulted in the formation of alumina support-bound surface species only: linear adsorbed CO2, bidentate carbonates and bicarbonates. Room temperature exposure of all three samples to CO produced IR features characteristic of both ionic and metallic Pd, as well as bands we observed upon CO2 adsorption (alumina support-bound species). Low temperature (100 K) adsorption of CO on the three samples provided information about the state of Pd after oxidation and reduction. Oxidized samples contained exclusively ionic Pd, while mostly metallic Pd was present in the reduced samples. Subsequent annealing of the CO-saturated samples revealed the facile (low temperature) reduction of PdOx species by adsorbed CO. This process was evidenced by the variations in IR bands characteristic of ionic and metallic Pd-bound CO, as well as by the appearance of IR bands associated with CO2 adsorption as a function of annealing temperature. Samples containing oxidized Pd species (oxidized, annealed or reduced) always produced CO2 upon their exposure to CO, while no CO2-related surface entities were observed on samples having only fully reduced (metallic) Pd.

 Please wait while we load your content...
Please wait while we load your content...