Is the contribution of cis and trans protonated 5-methylcytosine-SO3− isomers equal in the conversion to thymine-SO3− under bisulfite conditions? A theoretical perspective†
Abstract
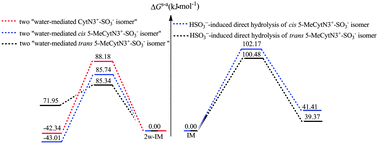
Cytosine (Cyt) can be converted to 5-methylcytosine (5-MeCyt) in CpG sequences of DNA. Conventional bisulfite sequencing can discriminate Cyt from 5-MeCyt, however inappropriate conversion of 5-MeCyt to thymine and a failure to convert Cyt to uracil always occur when Cyt and 5-MeCyt are treated with bisulfite, which would lead to erroneous estimates of DNA methylation densities. Here, the direct hydrolytic deamination of cis (paths A–C) and trans (paths A′–C′) 5-MeCytN3+-SO3− isomers with bisulfite have been explored at the MP2/6-311++G(3df,3pd)//B3LYP/6-311++G(d,p) level. The activation free energies (ΔGs-a≠) of the cis and trans 5-MeCytN3+-SO3− isomers’ paths exhibit no obvious differences, implying both isomers may make an equal contribution to the hydrolytic deamination of 5-MeCyt under bisulfite conditions. It is greatly expected that these results could aid experimental scientists to explore new methods to avoid the formation of the deaminated reactants (5-MeCytN3+-SO3−). Meanwhile, the HSO3−-induced direct hydrolytic deamination of cis and trans 5-MeCytN3+-SO3− isomers is represented by paths A and A′, respectively, and has been further explored in the presence of two water molecules. It was found that the contribution of two water molecules renders the HSO3−-induced direct hydrolytic deamination of cis and trans 5-MeCytN3+-SO3− isomers by paths A and A′ favourable. In addition, the ΔGs-a≠ values (85.74–85.34 kJ mol−1) of the rate-limiting steps of the two water-mediated paths A and A′ are very close to that of the theoretical value for CytN3+-SO3− (88.18 kJ mol−1), implying that the free barrier gap between Cyt and 5-MeCyt is very small under bisulfite conditions. This further suggests that bisulfite sequencing technology may be easily influenced by the external environment.

 Please wait while we load your content...
Please wait while we load your content...