Curie-type paramagnetic NMR relaxation in the aqueous solution of Ni(ii)
Abstract
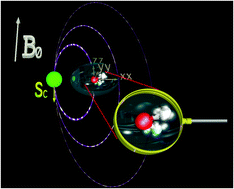
Ni2+(aq) has been used for many decades as a model system for paramagnetic nuclear magnetic resonance (pNMR) relaxation studies. More recently, its magnetic properties and also nuclear magnetic relaxation rates have been studied computationally. We have calculated electron paramagnetic resonance and NMR parameters using quantum-mechanical (QM) computation of molecular dynamics snapshots, obtained using a polarizable empirical force field. Statistical averages of hyperfine coupling, g- and zero-field splitting tensors, as well as the pNMR shielding terms, are compared to the available experimental and computational data. In accordance with our previous work, the isotropic hyperfine coupling as well as nuclear shielding values agree well with experimental measurements for the 17O nuclei of water molecules in the first solvation shell of the nickel ion, whereas larger deviations are found for 1H centers. We report, for the first time, the Curie-type contribution to the pNMR relaxation rate using QM calculations together with Redfield relaxation theory. The Curie relaxation mechanism is analogous to chemical shift anisotropy relaxation, well-known in diamagnetic NMR. Due to the predominance of other types of paramagnetic relaxation mechanisms for this system, it is possible to extract the Curie term only computationally. The Curie mechanism alone would result in around 16 and 20 s−1 of relaxation rates (R1 and R2 respectively) for the 1H nuclei of water molecules bonded to the Ni2+ center, in a magnetic field of 11.7 T. The corresponding 17O relaxation rates are around 33 and 38 s−1. We also report the Curie contribution to the relaxation rate for molecules beyond the first solvation shell in a 1 M solution of Ni2+ in water.
 Please wait while we load your content...
Please wait while we load your content...