Inner reorganization limiting electron transfer controlled hydrogen bonding: intra- vs. intermolecular effects†
Abstract
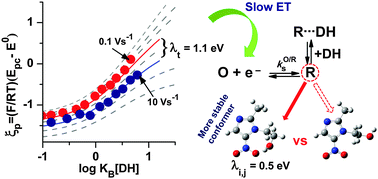
In this work, experimental evidence of the influence of the electron transfer kinetics during electron transfer controlled hydrogen bonding between anion radicals of metronidazole and ornidazole, derivatives of 5-nitro-imidazole, and 1,3-diethylurea as the hydrogen bond donor, is presented. Analysis of the variations of voltammetric EpIcvs. log KB[DH], where KB is the binding constant, allowed us to determine the values of the binding constant and also the electron transfer rate kO/Rs, confirmed by experiments obtained at different scan rates. Electronic structure calculations at the BHandHLYP/6-311++G(2d,2p) level for metronidazole, including the solvent effect by the Cramer/Truhlar model, suggested that the minimum energy conformer is stabilized by intramolecular hydrogen bonding. In this structure, the inner reorganization energy, λi,j, contributes significantly (0.5 eV) to the total reorganization energy of electron transfer, thus leading to a diminishment of the experimental kO/Rs.

 Please wait while we load your content...
Please wait while we load your content...