A fluorescence polarization assay for the experimental validation of an in silico model of the chemokine CXCL8 binding to receptor-derived peptides†
Abstract
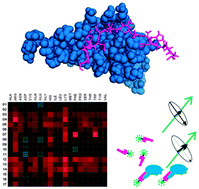
Peptide based inhibitors of protein–protein interactions are of great interest in proteomics, structural biology and medicinal chemistry. Optimized inhibitors can be designed by experimental approaches or by computational prediction. Ideally, computational models are adjusted to the peptide–protein complex of interest according to experimental data obtained in specific binding experiments. The chemokine CXCL8 (interleukin-8) is an interesting target for drug discovery due to its role in inflammatory diseases. Given the available structural data and information on its receptor interactions it constitutes a basis for the rational design of inhibitor peptides. Starting from the reported structure of CXCL8 in complex with a peptide derived from its receptor CXCR1 we developed a computational docking procedure to estimate the changes in binding energy as a function of individual amino acid exchanges. This indicates whether the respective amino acid residue must be preserved or can be substituted to maintain or improve affinity, respectively. To validate and improve the assumptions made in this docking simulation we established a fluorescence polarization assay for receptor-derived peptides binding to CXCL8. A peptide library was tested comprising selected mutants characterized by docking simulations. A number of predictions regarding electrostatic interactions were confirmed by these experiments and it was revealed that the model needed to be corrected for backbone flexibility. Therefore, the assay presented here is a promising tool to systematically improve the computational model by iterative cycles of modeling, experimental validation and refinement of the algorithm, leading to a more reliable model and peptides with improved affinity.

 Please wait while we load your content...
Please wait while we load your content...