Trapping of redox-mediators at the surface of Chlorella vulgaris leads to error in measurements of cell reducing power†
Abstract
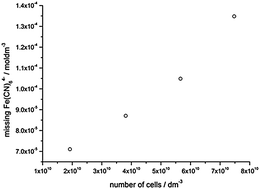
The reduction of the redox mediator ferricyanide, [Fe(CN)6]3−, by a range of algal and bacterial species, is frequently measured to probe plasma membrane ferrireductase activity or to quantify the reducing power of algal/bacterial biofilms and suspensions. In this study we have used rotating disk electrochemistry (RDE) to investigate the reduction of ferricyanide by the model organism Chlorella vulgaris. Importantly, we have seen that the diffusion limited current due to the oxidation of ferrocyanide, [Fe(CN)6]4−, at the electrode decreased linearly as C. vulgaris was added to the solution, even though in a pure ferrocyanide solution the algae are not able to reduce the mediator further and are simply spectator ‘particles’. We attribute this effect to trapping of ferrocyanide at the cell surface, with up to 14% of the ferrocyanide missing from the solution at the highest cell concentration. The result has important implications for all techniques that use electrochemistry and other concentration dependent assays (e.g. fluorescence and colourimetry) to monitor ferrocyanide concentrations in the presence of both biofilms and cell suspensions. Analyte trapping could lead to a substantial underestimation of the concentration of reduced product.

 Please wait while we load your content...
Please wait while we load your content...