Solvent-mediated crystal-to-crystal transformations from a cationic homometallic metal–organic framework to heterometallic frameworks†
Abstract
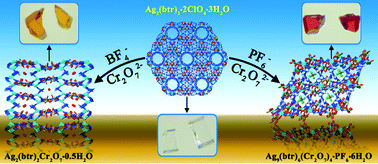
Two unprecedented heterometallic metal–organic frameworks (MOFs), Ag2(btr)2Cr2O7·0.5H2O (1) and Ag9(btr)6(Cr2O7)4·PF6·6H2O (2) [btr = 4,4′-bis(1,2,4-triazole)], were synthesized through crystal-to-crystal transformation when the monometallic MOF Ag2(btr)2·2ClO4·3H2O was immersed in the aqueous solutions of KPF6–K2Cr2O7 and NaBF4–K2Cr2O7, respectively. The transformation follows a solvent-mediated anion-induced mechanism through the dissolution–reaction–crystallization process. Single-crystal X-ray diffraction analyses reveal that both 1 and 2 are three-dimensional structures based on Ag+, Cr2O72− and btr. In 1, Cr2O72− adopts a bidentate bridging mode, and Ag+ ions are linked by Cr2O72− and btr into a neutral framework. However, Cr2O72− in 2 exhibits two types of unprecedented bridging modes through bridging four and five Ag+ ions. Ag+ ions in 2 are bridged by Cr2O72− and btr to form a cationic framework. The non-coordinated BF4−/PF6− anions show a structure-directing effect during the crystal-to-crystal transformations and can be considered as structure-directing agents. The second-harmonic-generation (SHG) measurement shows that 1 is a non-linear optical complex.

 Please wait while we load your content...
Please wait while we load your content...