Separation of planar rotamers through intramolecular hydrogen bonding in polysubstituted 5-nitrosopyrimidines†‡
Abstract
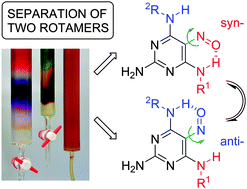
While purifying new polysubstituted 5-nitrosopyrimidines, the unique separation of pairs of rotamers as chemical species, stabilized by a single intramolecular hydrogen bond and differing only in nitroso group orientation, was achieved. This interesting stereochemical phenomenon is compared to the well-known atropisomerism.

 Please wait while we load your content...
Please wait while we load your content...