Modification of fulleropyrazolines modulates their cleavage by light†
Abstract
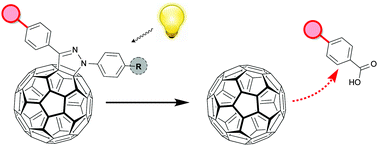
The extraordinary electrochemistry and the tunability of their energy levels allows the use of fulleropyrazolines in photovoltaics and charge-transfer systems. Here we show that substitution in position 1 tunes photolytic stability; electron-donating groups facilitate 1,3-dipolar cycloreversion to fullerene. This discovery has implications not only for photovoltaic stability but also highlights a potential strategy for photo-controlled fullerene release systems (‘photo-caged’/‘photo-activated’ fullerene).

 Please wait while we load your content...
Please wait while we load your content...