An organocatalytic one-pot cascade incorporating the Achmatowicz reaction affording 3-pyrone derivatives†
Abstract
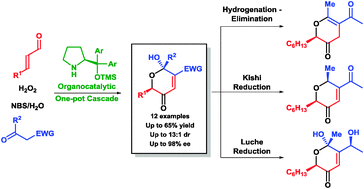
The development of an organocatalytic one-pot cascade for the annulation of simple starting materials: α,β-unsaturated aldehydes, hydrogen peroxide, β-carbonyl compounds and NBS to furnish optically active 3-pyrones in good yield and with excellent enantioselectivity is presented. Further diversification of the obtained products is demonstrated by selective reductive transformations.

 Please wait while we load your content...
Please wait while we load your content...