Autocatalytic one pot orchestration for the synthesis of α-arylated, α-amino esters†
Abstract
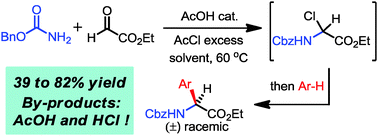
A novel acetyl chloride-mediated cascade transformation involving three components (benzyl carbamate, ethyl glyoxylate and arene nucleophiles) is reported. Aryl orthogonally protected α-amino acids are obtained in a one pot cascade, using a mild AcOH–AcCl system, via a critical autocatalytic dehydration-activation step ensuring an original and efficient Friedel–Crafts orchestration.

 Please wait while we load your content...
Please wait while we load your content...