Dearomatization of tryptophols via a vanadium-catalyzed asymmetric epoxidation and ring-opening cascade†
Abstract
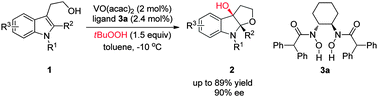
An enantioselective epoxidation of tryptophols followed by an intramolecular epoxide opening reaction was realized by chiral vanadium catalysts derived from C2 symmetric bis-hydroxamic acid (BHA) ligands. 3a-Hydroxyfuroindoline derivatives with up to 89% yield and 90% ee were obtained under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...