Electrochemical characterization of luminol and its determination in real samples
Abstract
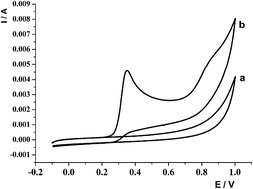
The electrochemical behavior of luminol, an important molecule in forensic science, was studied in Britton–Robinson buffer solution (pH 2–pH 13) at a glassy carbon electrode using cyclic voltammetry and differential pulse voltammetry techniques. Luminol showed a well-defined and irreversible oxidation peak at 0.33 V (vs. Ag/AgCl) in 0.05 mol L−1 Britton–Robinson buffer solution at pH 12. The electrooxidation process of luminol was adsorption-controlled and irreversible over the pH range studied (2.0–13). The differential pulse voltammetric determination of luminol was carried out. Under optimal conditions, the glassy carbon electrode displayed a linear response over the range from 1.8 × 10−8 to 4.5 × 10−4 mol L−1 luminol concentration, and statistical data were evaluated. A commercially available real sample that contained luminol was analyzed according to this method. The luminol percentage of the sample was obtained as 21.2 (±0.4)%.

 Please wait while we load your content...
Please wait while we load your content...