Sensitive electrochemical determination of yohimbine in primary bark of natural aphrodisiacs using boron-doped diamond electrode†
Abstract
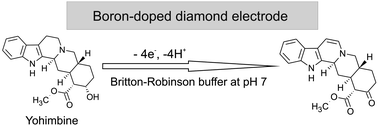
For the first time, a simple and sensitive analytical method for the direct determination of yohimbine is presented using differential pulse voltammetry with a boron-doped diamond electrode. Two irreversible oxidation peaks, a distinct one at +0.80 and a second poorly-defined one at +1.65 V, were observed when cyclic voltammetry was carried out in Britton–Robinson buffer solution at pH 7 (vs. Ag/AgCl). With optimized differential pulse voltammetric parameters (pulse amplitude 100 mV, pulse time 25 ms, step potential 5 mV and scan rate 10 mV s−1), the current response of yohimbine at +0.80 V was linearly proportional to the concentration in the range from 0.25 to 90.9 μmol L−1 with a low detection limit of 0.13 μmol L−1 (0.046 mg L−1) and a good repeatability (relative standard deviation of 2.5% at 18.4 μmol L−1 for n = 6). The practical applicability of the developed method was demonstrated by the assessment of the total content of yohimbine in extracts of the primary bark of natural aphrodisiacs such as Pausinystalia yohimbe and Rauvolfia serpentina with recoveries in the range of 92–97%. The proposed electrochemical procedure represents an inexpensive and effective analytical alternative for the quality control analysis of products containing yohimbine and other biologically and structurally related alkaloids used as natural dietary supplements.

 Please wait while we load your content...
Please wait while we load your content...