Utilization of facile synthesized Fe3O4 nanoparticles as a selective support for preconcentration of lead ions from food and environmental samples
Abstract
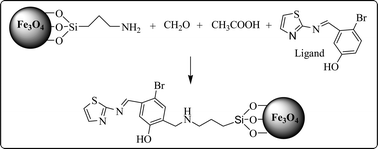
In this research, magnetic Fe3O4 nanoparticles were synthesized by a simple reaction system that takes 5 min at room temperature utilizing FeSO4 as the only iron source. Then their surface was modified with (3-aminopropyl)triethoxysilane and 4-bromo-3-(thiazol-2-ylimino)methylphenol using the Mannich reaction. Modified sorbent showed high selectivity for lead ions in the pH range of 6.5–8.0 as the relative selectivity factor (αr) of lead with respect to other cations was in the range of 150–1500. Fourier transform infrared, scanning electron microscopy, X-ray powder diffraction, vibrating sample magnetometry and elemental analysis techniques have been used for characterization of the prepared sorbent. The reusability of the sorbent was also investigated, and it was found that this sorbent is stable for up to eight adsorption–elution cycles without any noticeable drop-off in the recovery of lead ions.

 Please wait while we load your content...
Please wait while we load your content...