Self-healing polyacrylic acid hydrogels†
Abstract
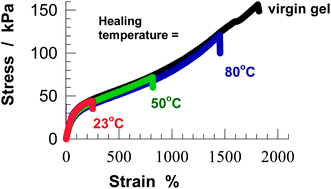
pH responsive physical gels were prepared by micellar copolymerization of acrylic acid with 2 mol% stearyl methacrylate (C18) in a solution of worm-like sodium dodecyl sulfate micelles. The physical gels are insoluble in water and exhibit time-dependent dynamic moduli, high Young's modulus (6–53 kPa), high fracture stress (41–173 kPa), high elongation ratios at break (1800–5000%), and self-healing, as evidenced by rheological and mechanical measurements. Cyclic elongation tests show significant hysteresis due to the existence of reversibly breakable bonds (up to 25%) within the gel network. As the polymer concentration is increased, both the lifetime of hydrophobic associations and the mechanical strength of gels increase while the elongation ratio at break decreases. As compared to the polyacrylamide gels formed under identical conditions, the present hydrogels exhibit a much stronger self-healing effect, which is attributed to the cooperative hydrogen bonding between the carboxyl groups stabilizing the hydrophobic domains. The efficiency of self-healing significantly increases with increasing temperature due to the decrease of the lifetime of hydrophobic associations.

 Please wait while we load your content...
Please wait while we load your content...