Exploring the role of ion solvation in ethylene oxide based single-ion conducting polyanions and polycations
Abstract
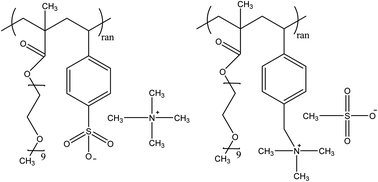
Ionomers with short ethylene oxide side chains are synthesized by free radical polymerization; to systematically test effects of solvating anion and cation, including directly substituting the ion attached to the polymer with its counterion, without solvent. Dielectric relaxation spectroscopy is used to measure the conductivity and dielectric constant in these ionomers, and the electrode polarization at very low frequencies is used to assess the number density of simultaneously conducting ions and their mobility. Conducting ion content and room temperature conductivity are larger for polyanions than their corresponding polycation because the cationic counterion can be more effectively solvated by the ether oxygen atoms compared to the sterically hindered polycation. Changing ester linkages to amide linkages in polycations boosts conducting ion content and static dielectric constant at high ion content by –NH solvating the anionic counterion. Such findings point a clear path toward design of superior single-ion conductors for a myriad of energy materials applications.

 Please wait while we load your content...
Please wait while we load your content...