Several rare earth coordination compounds and the first actinide coordination compound of the recently introduced multifunctional ligand (S)P[N(Me)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)Py]3 (1, Py = pyridyl) have been synthesized and characterized. The electronic and structural properties of these complexes were probed by X-ray diffraction analysis, X-ray absorption fine structure (XAFS), and advanced nuclear magnetic resonance (NMR) spectroscopy. Pulsed field-gradient spin-echo (PGSE) diffusion measurements and 1H,19F heteronuclear Overhauser spectroscopy (HOESY) revealed that the degree of ion pairing of the trivalent rare earth complexes [Ln(1)(OTf)3] (Ln = Y (2), La (3), Sm (4), and Lu (5); [OTf]− = [O3SCF3]−) depends on their metal cation ionic radii and decreases in acetonitrile solution for the smaller lanthanides. The plutonium(III) complex 6 exhibits, however, a significantly different behavior in solution and has a much stronger tendency to form solvent-separated ion pairs.
C(H)Py]3 (1, Py = pyridyl) have been synthesized and characterized. The electronic and structural properties of these complexes were probed by X-ray diffraction analysis, X-ray absorption fine structure (XAFS), and advanced nuclear magnetic resonance (NMR) spectroscopy. Pulsed field-gradient spin-echo (PGSE) diffusion measurements and 1H,19F heteronuclear Overhauser spectroscopy (HOESY) revealed that the degree of ion pairing of the trivalent rare earth complexes [Ln(1)(OTf)3] (Ln = Y (2), La (3), Sm (4), and Lu (5); [OTf]− = [O3SCF3]−) depends on their metal cation ionic radii and decreases in acetonitrile solution for the smaller lanthanides. The plutonium(III) complex 6 exhibits, however, a significantly different behavior in solution and has a much stronger tendency to form solvent-separated ion pairs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)Py]3 (1, Py =
C(H)Py]3 (1, Py = 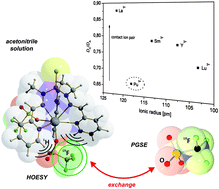

 Please wait while we load your content...
Please wait while we load your content...