Separation of enantiopure m-substituted 1-phenylethanols in high space-time yield using Bacillus subtilis esterase†
Abstract
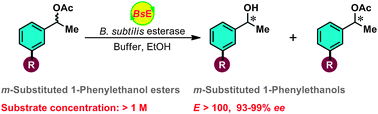
A recombinant Bacillus subtilis esterase (BsE) expressed in E. coli was found to exhibit excellent enantioselectivity (E was always greater than 100) towards m-substituted 1-phenylethanol acetates in the enantioselective hydrolysis reaction. An explanation for the high enantioselectivity observed towards these substrates was provided by molecular modeling. Moreover, the BsE also showed strong tolerance towards a high concentration of m-substituted 1-phenylethanol acetates (up to 1 M). Based on these excellent catalytic properties of BsE, a kind of m-substituted 1-phenylethanols,

 Please wait while we load your content...
Please wait while we load your content...