Phototransformation of azoxystrobin fungicide in organic solvents. Photoisomerization vs. photodegradation
Abstract
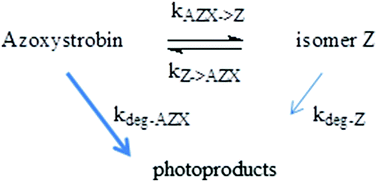
Azoxystrobin is a systemic fungicide that has a tendency to accumulate at the surface of crop leaves or inside their cuticle where it undergoes photodegradation. Its photochemistry was investigated in n-heptane and isopropanol to mimic the polarity of wax leaves. Using analytical and kinetic data, we demonstrate that azoxystrobin (isomer E) undergoes efficient photoisomerization into the isomer Z with a quantum yield of 0.75 ± 0.08. This value is 30-fold higher than that reported in aqueous solution. The photoisomerization of isomer Z into azoxystrobin is more efficient with a chemical yield of 0.95 ± 0.1. In addition, a pseudo photostationary equilibrium is reached when the ratio [azoxystrobin]/[isomer Z] is 2.0 ± 0.1. Photodegradation also takes place from azoxystrobin (quantum yield = 0.073 ± 0.008). Photoproducts mainly arise from bond cleavage between rings and from demethylation of the ether with or without saturation of the acrylate double bond. Theoretical calculations were undertaken to investigate the photoisomerization mechanism and the solvent effect. These data show that the photochemical reactivity of azoxystrobin is enhanced when the solvent polarity decreases and thus should be significant in leaf waxes.

 Please wait while we load your content...
Please wait while we load your content...