Photolysis of cycloxydim, a cyclohexanedione oxime herbicide. Detection, characterization and reactivity of the iminyl radical
Abstract
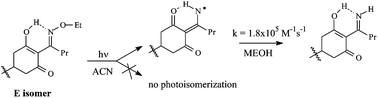
Cyclohexanedione oxime herbicides have been reported to be readily photodegraded in the environment but the reaction mechanism has never been studied in detail. Here we investigated the photolysis of cycloxydim (CD) in acetonitrile and water, solvents in which CD is present as two distinct tautomeric forms: keto form in water and enol form with an intramolecular hydrogen bond between the enolic proton and the nitrogen atom of the oxime in acetonitrile O–H⋯N. CD (E isomer) undergoes photoisomerization in water but not in acetonitrile. This difference is attributed to the inhibiting effect of the intramolecular hydrogen bond existing in acetonitrile. In both solvents, irradiation of CD leads to the cleavage of the N–O bond as evidenced by the imine formation. The iminyl radical could be detected in acetonitrile by nanosecond laser-flash photolysis (λmax = 280, 320 and 480 nm, τ ∼ 100 μs). This radical is unreactive toward oxygen but readily abstracts an H atom from methanol (k = 1.8 × 105 M−1 s−1). Quantum calculations confirm the assignment of the transient species to the iminyl radical by showing that (i) the most stable structure of the iminyl carries a large spin density on the ring carbon and on the nitrogen atoms, (ii) the enolic H atom is transferred to the nitrogen atom and (iii) the intramolecular hydrogen bond O⋯H–N is responsible for both the iminyl long wavelength absorption and its high hydrogen abstraction reactivity.

 Please wait while we load your content...
Please wait while we load your content...