Diastereoselective oxidative α-amination of aliphatic aldehydes catalyzed by iodine: synthesis of syn-γ-hydroxy-α-amino acetals†
Abstract
Aldehydes can react with secondary amines to give α-amino acetals via the α-amination of aliphatic aldehydes catalyzed by iodine. The presence of an asymmetric hydroxylated center at the γ-position of the aldehyde was found to induce the stereoselective amino group. This method represents a stereoselective α-amination of γ-hydroxyaldehydes for the synthesis of syn-γ-hydroxy-α-amino acetals in good yields and reasonable diastereoselectivities under very mild conditions.
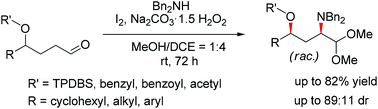

 Please wait while we load your content...
Please wait while we load your content...