Discovery of 4,6-substituted-(diaphenylamino)quinazolines as potent c-Src inhibitors
Abstract
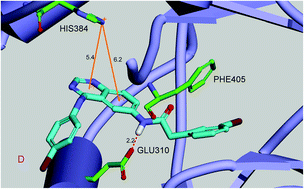
A series of 4,6-substituted-(diaphenylamino)quinazolines as c-Src inhibitors have been prepared and their biological activity has also been evaluated. All the compounds displayed potential antiproliferation activities, with IC50 values ranging from 3.42 μM to 118.81 μM in five human tumor cell lines. Particularly, compound 15 exhibited higher cytotoxicity against the tested five tumor cell lines compared to the other small molecules. Generally, most of these compounds showed selectivity between the A549 cells and the other four cells, according to their corresponding IC50 values. The results obtained from the in vitro enzyme assay indicated compound 15 has remarkable inhibitory activity against c-Src kinase with an IC50 value of 27.3 nM, which is comparable to the control compounds. Furthermore, molecular docking and QSAR study by means of DS 3.5 (Discovery Studio 3.5, Accelrys, Co. Ltd) explored the binding modes and the structure and activity relationship (SAR) of these derivatives.

 Please wait while we load your content...
Please wait while we load your content...