Catalytic synthesis of enantiopure mixed diacylglycerols – synthesis of a major M. tuberculosis phospholipid and platelet activating factor†
Abstract
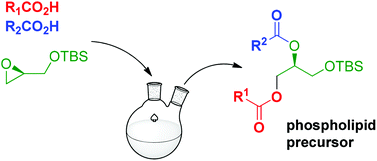
An efficient catalytic one-pot synthesis of TBDMS-protected diacylglycerols has been developed, starting from enantiopure glycidol. Subsequent migration-free deprotection leads to stereo- and regiochemically pure diacylglycerols. This novel strategy has been applied to the synthesis of a major Mycobacterium tuberculosis phospholipid, its desmethyl analogue, and platelet activating factor.

 Please wait while we load your content...
Please wait while we load your content...