Aromatic thioglycoside inhibitors against the virulence factor LecA from Pseudomonas aeruginosa†
Abstract
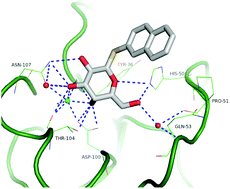
Three small families of hydrolytically stable thioaryl glycosides were prepared as inhibitors of the LecA (PA-IL) virulence factor corresponding to the carbohydrate binding lectin from the bacterial pathogen Pseudomonas aeruginosa. The monosaccharidic arylthio β-D-galactopyranosides served as a common template for the major series that was also substituted at the O-3 position. Arylthio disaccharides from lactose and from melibiose constituted the other two series members. In spite of the fact that the natural ligand for LecA is a glycolipid of the globotriaosylceramide having an α-D-galactopyranoside epitope, this study illustrated that the β-D-galactopyranoside configuration having a hydrophobic aglycon could override the requirement toward the anomeric configuration of the natural sugar. The enzyme linked lectin assay together with isothermal titration microcalorimetry established that naphthyl 1-thio-β-D-galactopyranoside (11) gave the best inhibition with an IC50 twenty-three times better than that of the reference methyl α-D-galactopyranoside. In addition it showed a KD of 6.3 μM which was ten times better than that of the reference compound. The X-ray crystal structure of LecA with 11 was also obtained.

 Please wait while we load your content...
Please wait while we load your content...