Acid-catalyzed reactions of 3-hydroxy-2-oxindoles with electron-rich substrates: synthesis of 2-oxindoles with all-carbon quaternary center†‡
Abstract
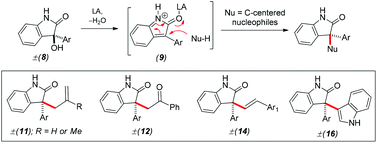
A Lewis acid catalyzed reaction of in situ generated 2H-indol-2-one (9) with various 2π and other electron-rich substrates has been developed. A variety of electron-rich substrates such as allyl/methallyltrimethylsilane, phenylacetylene, styrenes, acetophenone, and indoles have been used. The methodology provides a straightforward approach for the synthesis of 2-oxindoles with an all-carbon quaternary center at the pseudobenzylic position.

 Please wait while we load your content...
Please wait while we load your content...