Quadruply hydrogen-bonded heteroduplexes based on imide and urea units arrayed with ADDA/DAAD sequences†
Abstract
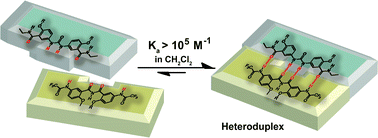
A new class of imide- and urea-based hetero-strands with a quadruple ADDA/DAAD hydrogen-bond array was designed and synthesized from easily accessible starting materials. The molecular recognition between the two different strands depends highly on the substituents and the linker between neighboring hydrogen-bonds, which results in the stability of these heteroduplexes varying from 103 to >105 M−1 in apolar solvents. In particular, an increase of the association constant by up to one order of magnitude was observed by derivatizing the ADDA arrays at the termini with electron-withdrawing groups. Molecular modelling of the representative complementary complexes reveals the binding mode of four hydrogen-bond arrays that agrees with the matched pair.

 Please wait while we load your content...
Please wait while we load your content...