Asymmetric organocatalytic Strecker-type reactions of aliphatic N,N-dialkylhydrazones†
Abstract
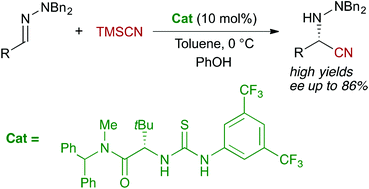
The enantioselective organocatalytic Strecker-type reaction of aliphatic N,N-dialkylhydrazones is presented. Using trimethylsilyl cyanide (TMSCN) as the cyanide source, the reaction can be efficiently catalyzed by a tert-leucine-derived bifunctional thiourea to afford the corresponding hydrazino nitriles in good to excellent yields (50–96%) and moderate to good enantioselectivities, up to 86% ee. Further transformations of the nitrile functionality allow access to useful protected hydrazino acids and imidazolidinones. Interestingly, some of the hydrazino nitriles and their derivatives could be recrystallized in high recovery, yielding essentially pure enantiomers.

 Please wait while we load your content...
Please wait while we load your content...