Constructions of tetrahydro-γ-carboline skeletons via intramolecular oxidative carbon–carbon bond formation of enamines†
Abstract
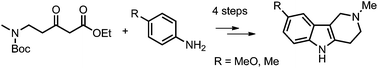
The synthetically and biologically important 4-methyl and 4-methoxy tetrahydro-γ-carboline compounds were readily synthesized in high yields from an aryl amine and a 5-amino-3-oxopentanoate derivative through a series of reactions of enamination, oxidative annulation, deprotection/lactamization and the final reduction reaction of the

 Please wait while we load your content...
Please wait while we load your content...