Mixed-solvothermal synthesis, structures, luminescent and surface photovoltage properties of four new transition metal diphosphonates with a 3D supramolecular structure†
Abstract
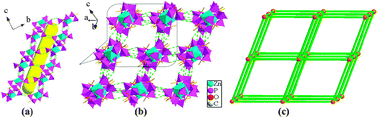
Four new transition metal(II) diphosphonates with a 3D supramolecular structure, M(hedpH2)3·3NH2(CH3)2NH(CH3)3·3H2O (M = Mn (1), Co (2), Ni (3), Zn (4); hedpH4 = 1-hydroxyethylidenediphosphonate acid) have been synthesized under mixed-solvothermal conditions and structurally characterized. Compounds 1–4 are isomorphous and adopt a three-dimensional supramolecular network structure containing {M(hedpH2)3}4− cluster units. The interconnection of {MO6} and {CPO3} polyhedra via corner-sharing forms a {M(hedpH2)3}4− cluster, and these isolated clusters are extended by hydrogen bonds to form a two-dimensional layer structure, which are further connected through hydrogen bonding interactions to give rise to a 3D supramolecular structure. Surface

 Please wait while we load your content...
Please wait while we load your content...