Silver(i) coordination chemistry: from 1-D chains to molecular rectangles†
Abstract
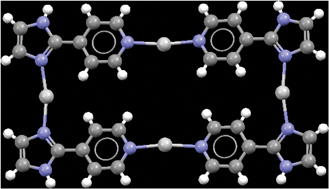
A series of silver(I) coordination compounds with the ligand 2-(4-pyridyl)imidazole 4-PyIm has been synthesized and crystallographically characterized in order to explore the role of counterions, solvents and ligand charge in the resulting supramolecular architectures. With perchlorate and hexafluorophosphate, two iso-structural 1-D coordination-polymers were obtained, but when changing the counterion to nitrate and using water as solvent, an unusual molecular rectangle composed of four ligands and four Ag(I) ions was obtained, and the assembly was controlled by hydrogen bonding via the anion and water molecules. Finally, when the ligand was deprotonated, the change in the number of binding sites (from two to three) was reflected in an increase in the coordination number for the silver ion.

 Please wait while we load your content...
Please wait while we load your content...