In vivo investigation of the substrate recognition capability and activity affecting amino acid residues of glycosyltransferase FscMI in the biosynthesis of candicidin†
Abstract
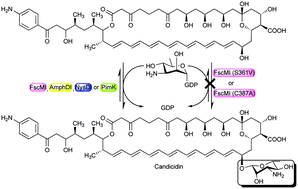
Alteration of sugar moieties of natural products often leads to novel antibiotics with different chemical and physical properties. fscMI is a putative glycosyltransferase (GT) in a gene cluster for the production of candicidin, a polyene macrolide antibiotic, produced by Streptomyces sp. FR-008. In this report, we established an in vivo biochemical detection system by inactivating fscMI and the DH11 domain of polyketide synthase (PKS) through double homologous recombination to unveil the interaction between polyene GTs and their substrates. We found that homologous GT genes including amphDI, nysDI and pimK can catalyze the conversion of candicidin aglycone into candicidin/FR-008-III in fscMI mutant, suggesting that homologous polyene GTs show some tolerance toward aglycones and that it is possible to create new polyene analogues with altered aglycones through genetic engineering. Inactivation of the DH11 domain of PKS led to novel polyene derivatives with mycosamine added to the altered polyketide backbones, further confirming the loose substrate specificity of polyene GTs. Furthermore, mutation of Ser346, Ser361, His362 or Cys387 of FscMI by site-directed mutagenesis significantly reduced its catalytic activity. Further analysis suggested that Ser361 and Cys387 are likely the critical donor interacting residues that could affect the activity of GT FscMI. To our knowledge, this is the first report of the critical residues in a polyene GT.

 Please wait while we load your content...
Please wait while we load your content...