Theoretical studies of iron(iii)-catalyzed intramolecular C–H amination of azides†
Abstract
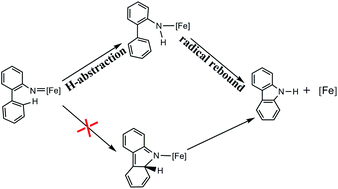
Density functional theory calculations have been carried out to study the reaction mechanism of the [FeIII(F20TPP)Cl] catalyzed C–H amination reaction. The calculations show that the classical three-step mechanism for other metals (Ru, Rh, Ir and Zn), including N2 liberation, C–N bond formation and 1,2-hydrogen shift, does not fit the iron(III)-catalyzed system. After N2 liberation, the favorable reaction pathway for the iron(III)-catalyzed system is a 1,2-hydrogen shift preceding C–N bond formation, i.e., a H-abstraction/radical rebound mechanism.

 Please wait while we load your content...
Please wait while we load your content...