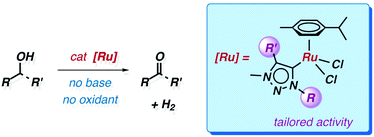
Wingtip substituents tailor the catalytic activity of ruthenium triazolylidene complexes in base-free alcohol oxidation†
Abstract
A series of RuII (η6-arene) complexes with 1,2,3-triazolylidene
- This article is part of the themed collection: N heterocyclic carbenes

 Please wait while we load your content...
Please wait while we load your content...