How does the addition of steric hindrance to a typical N-heterocyclic carbeneligand affect catalytic activity in olefin metathesis?†
Abstract
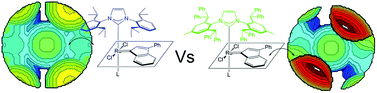
Density functional theory (DFT) calculations were used to predict and rationalize the effect of the modification of the structure of the prototype 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene)
- This article is part of the themed collection: N heterocyclic carbenes

 Please wait while we load your content...
Please wait while we load your content...