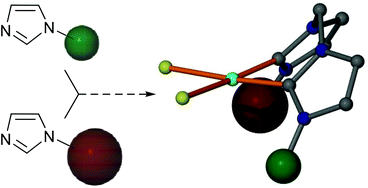
N,N′-Asymmetrically substituted, methylene-linked bis(imidazol-2-ylidene) complexes have been prepared subsequent to a selective synthesis of the bis(imidazolium) salt precursors involving the quarternisation of N-alkyl and -aryl imidazoles with N-halomethyl imidazolium salts. The adaptability of the ligand precursor synthesis is illustrated through access to the N-Me/N′-Mes and N-Mes/N′-2,6-(i-Pr)2Ph systems, leading to the Pd(II) complexes [{(MeIm)(MesIm)CH2}Pd(L)2]n+, L = Cl/I (n = 0) and NCMe (n = 2), and [{(MesIm)[2,6-(i-Pr)2PhIm]CH2}Pd(L)2], L = Cl/I. The dicationic hybrid N,N′-alkyl/aryl complex was inactive in the copolymerisation of ethylene/carbon monoxide, displaying reactivity akin to N,N′-dialkyl analogues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...