Syntheses, characterization and energetic properties of closo-(B12H12)2− salts of imidazolium derivatives†
Abstract
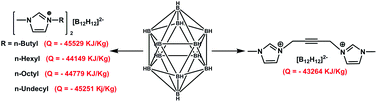
The diimidazolium derivative of acetylene and its salt 3,3′-(but-2-yne-1,4-diyl)bis(1-methyl-1H-imidazol-3-ium)chloride (1) was synthesized by a solvent free sonochemical method and then the counter chloride ions were replaced by closo-dodecaborate [(B12H12)2−] and perchlorate (ClO4−) anions respectively. Along with these two ionic salts, a series of salts with closo-dodecaborate and alkyl imidazolium cations were also synthesized. All the compounds were characterized by NMR and MASS spectral data, elemental analyses and thermogravimetric analyses. In addition to that enthalpy of combustion, enthalpy of formation and heat of explosion of all the compounds were experimentally determined. Based on the properties of these compounds, they can be used as insensitive energetic materials in various fields in propellant research and technology such as solid rocket propellants and burn rate accelerators.

 Please wait while we load your content...
Please wait while we load your content...