Two-dimensional stimulated resonance Raman spectroscopy study of the Trp-cage peptide folding†
Abstract
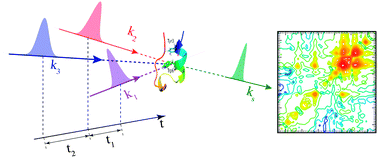
We report a combined molecular dynamics (MD) and ab initio simulation study of the ultrafast broadband ultraviolet (UV) stimulated resonance Raman (SRR) spectra of the Trp-cage mini protein. Characteristic two dimensional (2D) SRR features of various folding states are identified. Structural fluctuations erode the cross peaks and the correlation between diagonal peaks is a good indicator of the α-helix formation.

 Please wait while we load your content...
Please wait while we load your content...