Preparation of water-dispersible TiO2 nanoparticles from titanium tetrachloride using urea hydrogen peroxide as an oxygen donor†
Abstract
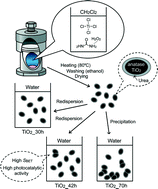
TiO2 nanoparticles were prepared from titanium tetrachloride (TiCl4) in CH2Cl2 at 80 °C for 30 h, 42 h and 70 h using urea hydrogen peroxide (UHP) as an oxygen donor with a TiCl4 : UHP molar ratio of 1 : 2. The XRD patterns and Raman spectroscopy results showed that the products consisted of anatase TiO2. IR and solid-state 13C NMR with cross polarization and magic angle spinning techniques revealed the presence of urea. TEM observation revealed that the products prepared by the reactions for 30 and 42 h consisted of water-dispersible spheroid nanoparticles with a long axis of ~5 nm, while an aggregation of nanoparticles was evident upon reaction for 70 h. Thermogravimetry, inductively-coupled plasma emission spectrometry and CHN analysis showed that the amount of urea increases in the following order: TiO2_42h, TiO2_70h, TiO2_30h. The photocatalytic activity of the products dispersible in water (TiO2_30h and TiO2_42h) was estimated based on the degradation behaviour of methylene blue, and TiO2_42h showed higher photocatalytic activity than TiO2_30h. It is proposed that TiCl4 was directly oxidized by UHP to form anatase TiO2 in the early stage of the process.

 Please wait while we load your content...
Please wait while we load your content...