Ion-induced diversity in structure and magnetic properties of hexacyanometalate–lanthanide bimetallic assemblies†
Abstract
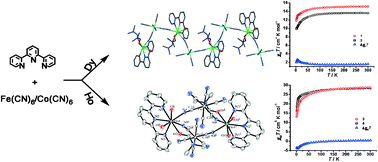
The reaction of trivalent DyIII/HoIII, 2,2′:6′,2′′-terpyridine (terpy), and hexacyanometallate [MIII(CN)6]3− (M = FeIII and CoIII) leads to the formation of a series of cyano-bridged complexes (1–4). In the case of DyIII, one-dimensional (1-D) alternating chains [DyIII(terpy)(DMF)2(H2O)2][MIII(CN)6]·3H2O (M = FeIII for 1 and CoIII for 2) were obtained, where each [MIII(CN)6]3− entity acts as a bis-monodentate bridging ligand towards two DyIII ions through two of its six cyanide groups in cis positions, respectively. Contrarily, in the case of HoIII, a molecular rectangle {[HoIII(terpy)(DMF)(H2O)2][MIII(CN)6]}2·nH2O·2CH3OH {(M = FeIII (3) and CoIII (4); and n = 6 or 7} has been formed although the synthesis method is same as for 1 and 2. Interestingly, solid-state direct-current magnetic susceptibility analyses demonstrate competing ferromagnetic interactions for 1 while complex 3 shows antiferromagnetic interactions. The variable-temperature magnetic behaviours observed for 2 and 4 only originate from isolated Dy and Ho ions, since a diamagnetic CoIII metal ion links the magnetic LnIII ions.

 Please wait while we load your content...
Please wait while we load your content...