Salt-bridge–π (sb–π) interactions at work: associative interactions of sb–π, π–π and anion–π in Cu(ii)-malonate–2-aminopyridine–hexafluoridophosphate ternary system†
Abstract
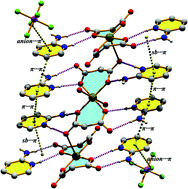
An essentially unexplored noncovalent interaction involving aromatic rings is re-defined and described: the salt-bridge–π interaction. It consists of the stacking interaction between an aromatic ring and a planar salt-bridge. If the aromatic ring is located under the cation of the salt-bridge, the interaction must be described as a cation–π interaction. Similarly, if the aromatic ring is located under the anion of the salt-bridge, the interaction must be described as an anion–π interaction. However, if the aromatic ring is just in the middle of both, a new definition of noncovalent bonding is required. Herein, we propose the term “salt-bridge–π (sb–π) interaction” to describe the stacking between an aromatic ring and a planar salt-bridge (for example, guanidinium and carboxylate ion pair). We also report the synthesis and X-ray characterization of one Cu(II) malonate complex with protonated 2-aminopyridine as the auxiliary ligand, which is acting as the counter cation, namely, {(C5H7N2)6[Cu(C3H2O4)2(H2O)2][Cu(C3H2O4)2](PF6)2}n (A) (C5H7N2 = protonated 2-aminopyridine, C3H4O4 = malonic acid) where this type of interaction is observed. Other weak forces like hydrogen bonding, π⋯π stacking and anion⋯π interactions were also found to be responsible for the overall stabilisation of the complex A. Interestingly, an extended supramolecular network of the type sb–π/π–π/π–π/π–anion has been observed in the solid state structure of complex A. This outstanding network of weak forces was observed for the first time in the crystalline structures of metal–organic hybrid complexes. From this perspective, the self-assembly process appears to be of great importance in this complex. The analysis of the crystalline structure of A with an emphasis on exploring this rare supramolecular network is presented here. The theoretical study combines the energetic analysis of the noncovalent forces that participate in the extended supramolecular network and the characterization of the different interactions by means of Bader's theory of “atoms in molecules”. We also present here Hirshfeld surface analysis to investigate the close intermolecular contacts.

 Please wait while we load your content...
Please wait while we load your content...