Structural study of four benzyloxybenzoic acids and benzyloxyanilines using X-ray powder diffraction: interplay of strong and weak intermolecular interactions†
Abstract
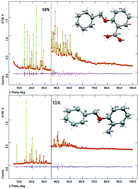
Crystal structures of two benzyloxybenzoic acids (1 and 2) and two benzyloxyanilines (3 and 4) have been solved from laboratory

 Please wait while we load your content...
Please wait while we load your content...