Synthetic methods and structural study of coordination polymers of Cd(ii) and Co(ii) with tetrathiafulvalene–tetracarboxylate†
Abstract
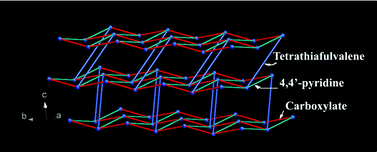
A series of coordination polymers of cadmium(II) and cobalt(II) with TTF–tetracarboxylate ligand (L) were prepared using room temperature synthetic methods and solvothermal method. The room temperature methods are subdivided to controlled evaporation method and two layers diffusion method. The fundamental structures of these compounds are {[M(L)0.5(B)(S1)x]·yS2}n (1–6) (M = Cd(II) or Co(II), B (base) = 2,2′-bpy, phen or 4,4′-bpy, S1 (coordinated solvent) = C2H5OH or H2O, and S2 (co-crystallized solvent) = H2O or CH3OH, x = 1 or 2, and y = 0 to 3). Compound 7 is a known ionic pair n[Co(4,4′-)2(H2O)4]·[Co(L)(H2O)2]n. At room temperature, no matter what the metal ions are, Cd(II) or Co(II), and the synthetic methods are, controlled evaporation or two layers diffusion, the products 1–4 and 7 are 1-D coordination polymers. Both the 2-D Co(II) coordination polymer 5 and 3-D coordination polymer 6 were prepared by solvothermal method. A new type of coordination mode of the TTF–tetracarboxylate ligand is found in the structures of 2 and 3, in which two carboxyl groups have a μ2-η2:η1 bridging mode, and the other two carboxyl groups are not coordinated. Electrochemical properties of the solid-state compounds were also investigated by cyclic voltammetry using surface-modified electrodes.

 Please wait while we load your content...
Please wait while we load your content...