Aldol reactions mediated by a tetrahedral boronate†
Abstract
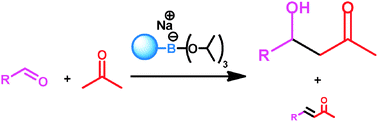
The base is a key factor in aldol reactions in organic media, determining the selectivity. Here, we describe a tetrahedral phenylboronate salt as a mild non-nucleophilic base that is able to catalyse the aldol reaction and significantly decrease the formation of undesired elimination products.

 Please wait while we load your content...
Please wait while we load your content...