β-Oligofurans†
Abstract
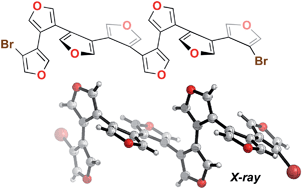
An iterative cross-coupling strategy has led to the first synthesis of the β-oligofuran family. Every member of the series of dibrominated analogues has been accessed from the bifuran to the octafuran, along with representative diiodides, non-halogenated congeners and cyclic analogues. Solution phase

 Please wait while we load your content...
Please wait while we load your content...