Driving selective aerobic oxidation of alkyl aromatics by sunlight on alcohol grafted metal hydroxides†
Abstract
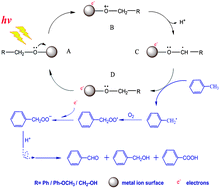
It is very difficult to selectively oxidise stable compounds such as
* Corresponding authors
a
School of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology, Brisbane, Qld 4001, Australia
E-mail:
hy.zhu@qut.edu.au
Fax: +61 7 3138 1804
Tel: +61 7 3138 1581
b
Institute of Chemistry, The Chinese Academy of Science, Beijing 100080, China
E-mail:
jczhao@iccas.ac.cn
Fax: +86 10 8261 6495
Tel: +86 10 8261 6495
c
Department of Chemistry, University of Western Ontario, London, Ontario, Canada
E-mail:
yhuang@uwo.ca
Fax: +01 519 661 2111
Tel: +01 519 661 2111 ext 86384
d
Institute of New Energy Material Chemistry, Nankai University, Tianjin 300071, China
E-mail:
guoranli@nankai.edu.cn
Fax: +86 22 2350 0876
Tel: +86 22 2350 0876
It is very difficult to selectively oxidise stable compounds such as

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
S. Sarina, H. Zhu, Z. Zheng, S. Bottle, J. Chang, X. Ke, J. Zhao, Y. Huang, A. Sutrisno, M. Willans and G. Li, Chem. Sci., 2012, 3, 2138 DOI: 10.1039/C2SC20114C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
