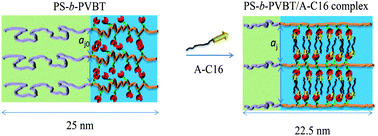
In this study we used nitroxide-mediated radical polymerization to synthesize poly(styrene-b-4-vinylbenzyl azide) (PS-b-PVBN3) and then used click chemistry to react it with propargyl-thymine (PT) to obtain a series of thymine (T)-containing block copolymers, poly(styrene-b-4-vinylbenzyl triazolylmethyl methylthymine) (PS-b-PVBT). We then added an amphiphilic surfactant, hexadecyladenine (A-C16), to complex with the PVBT units in the PS-b-PVBT copolymers through complementary multiple hydrogen bonding interactions. The resulting supramolecular comb–coil diblock copolymers formed lamellae-within-lamellae self-assembled structures, with PS lamellar domains (diameter: ca. 20–25 nm) in a matrix consisting of lamellar mesophases (lamellar inter-distance: ca. 2.3 nm) organized by the PVBT/A-C16 complex. Fourier transform infrared spectroscopy provided evidence for multiple hydrogen bonding between the A-C16 and T groups of the PS-b-PVBT copolymers. The small-angle X-ray scattering patterns of these self-assembled supramolecular systems were very temperature-sensitive. A striking feature was the appearance of the ordered scattering of the lamellar structure at temperatures higher than the melting point of A-C16 (>120 °C), with A-C16 becoming fully miscible with both the PS and PVBT phases at higher temperature; A-C16 was miscible only with the PVBT domains at room temperature, thereby influencing the volume fraction of the PS segment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...