Cascade dearomatization of N-substituted tryptophols via Lewis acid-catalyzed Michael reactions†
Abstract
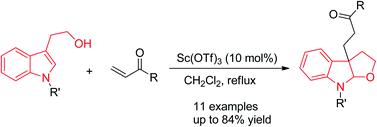
Lewis acid-catalyzed cascade dearomatization of N-substituted tryptophols via Michael addition reaction was developed. The generality of the method has been demonstrated by the synthesis of versatile furoindoline derivatives with a quaternary carbon center in good yields.

 Please wait while we load your content...
Please wait while we load your content...