Medium-sized and strained heterocycles from non-catalysed and gold-catalysed conversions of β-carbolines†
Abstract
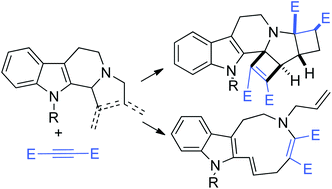
2-Allyl-1-vinyl-β-carbolines and dihydropyrrolo-β-carbolines react with activated internal alkynes through novel rearrangement reactions leading to complex polycyclic structures. Favored reaction pathways depend on reaction conditions and on the presence of gold catalysts. In particular, upon reaction with 2 equiv. of the alkyne, new hexacyclic structures 10 are formed with total stereocontrol.

 Please wait while we load your content...
Please wait while we load your content...