Fluorescent analogs of the marine natural product psammaplin A: synthesis and biological activity†
Abstract
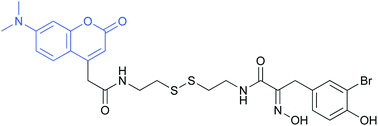
The symmetrical disulfide psammaplin A from the marine sponge Pseudoceratina sp. was synthesized and structurally altered by replacement of one of the α-(hydroxyimino)acyl units by a fluorescent 4-coumarinacetyl moiety. Thus, the first fluorescent analogs of psammaplin A were obtained. Structural variation also covered the substitution pattern of the phenyl ring. Cytotoxicity of psammaplin A against the mouse fibroblast cell line L-929 (IC50 0.42 μg mL−1) was about two-fold higher than that of the fluorescent hybrid compounds, whereas the disulfide containing two 4-coumarinacetyl moieties was inactive. Fluorescence microscopy revealed uptake of the 4-coumarinacetyl-α-(hydroxyimino)acyl hybrids into the cytoplasm leading to fluorescence in close proximity of the nuclear envelope, most likely in the Golgi apparatus. We did not observe strong fluorescence inside the nucleus, where most of the target histone deacetylases are located. We conclude that reduction of the disulfide probably takes place outside the nucleus. The psammaplin-derived thiol exhibited potent activity against histone deacetylase in the low nanomolar range, but diminished cytotoxicity. Antimicrobial activity of the new derivatives was also determined.

 Please wait while we load your content...
Please wait while we load your content...